K series x-rays are produced when an electron in the K (
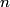 = 1) shell of one of the target atoms is knocked out of the atom by one of the fast electrons. The electrons in the K shell of nickel are bound by an energy of = 1) shell of one of the target atoms is knocked out of the atom by one of the fast electrons. The electrons in the K shell of nickel are bound by an energy of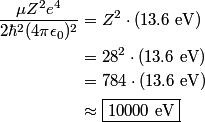 This is the energy that the fast electron must have to knock out a K shell electron. Therefore, answer (D) is correct. |